Abstract
Introduction: Given the availability of several active treatment (tx) options for CLL, providers frequently make tx recommendations based on their interpretation of objective data from consensus criteria and clinical trials. There are limited data describing patient (pt) experience, values, and insights when encountering multiple tx choices. The CLL Society, a pt-driven, physician-curated nonprofit organization focused on the unmet needs of the CLL community, sought to explore how CLL pts make tx decisions.
Methods: A 64 question survey was distributed online and in paper to CLL pts from Oct-Dec 2017. The survey was developed by CLL experts. The research was IRB-approved. Chi square was used for statistical comparison; all other analyses are descriptive in nature.
Results: 1147 pts from 48 states completed the survey. Median age was 65 (range 28-86), 46% male, 96% Caucasian.
Who Influences Tx: Of the 79% of pts who have seen a CLL expert, defined as a provider focused on CLL at an academic/research center, 95% rated their provider as very/extremely influential. 83% of pts rated their own opinions as influential, and 59% rated the opinion of a general hematologist/oncologist (gen heme/onc), a provider who sees a variety of cancer types, as influential. Only 27% let their provider unilaterally decide on tx. Of these pts, 19% trusted their provider to select the best tx and 5% didn't have enough understanding to be involved in the decision. 64% were influenced by outside sources of information, such as family members, live CLL support groups, or online sources including pt experts and forums.
Factors in Deciding Tx: Of the 67% pts surveyed who have been treated, 73% were offered > 1 tx choice by their provider. In deciding on a specific tx, 94% of respondents stated that it was very/extremely important to know there were tx options beyond their current regimen.
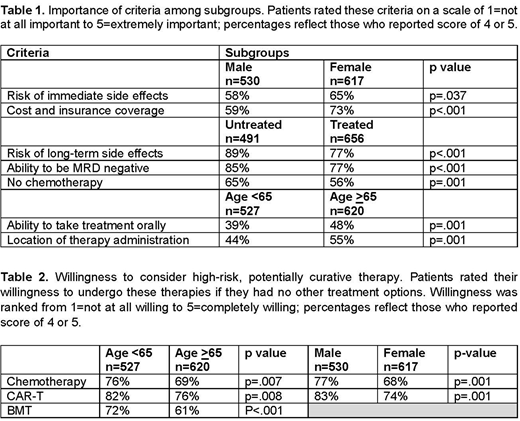
The most important factors in selecting tx were response rate (91%), overall survival (88%), progression-free survival (86%), long-term side effects (82%), ability to achieve undetectable MRD (80%), cost/insurance coverage (66%), and location of tx (50%) with significant differences between subgroups (Table 1).
Tx was impacted by cost or insurance issues for 16% (n=104). Of these 104 pts, cost/insurance issues resulted in tx delay for 34 and change in tx plan for 24 pts. 16 pts reported that an appeal led to coverage and 16 pts received financial assistance.
Pts' willingness to accept potentially curable but high-risk tx was heavily influenced by availability of alternate options. For example, 18% of pts would be willing to consider cytotoxic chemotherapy if alternate options were available vs. 72% if no options were available. Similar patterns were seen with CAR-T tx (28% vs. 79%) and bone marrow transplantation (BMT) (15% vs. 66%) with significant differences between subgroups (Table 2).
82% were willing to take a life-long tx for long-term control without potential for cure. Willingness to accept long-term tx was significantly higher in treated vs. untreated pts (84% vs. 77%, p=.006) and men vs. women (84% vs. 78%, p=.008).
Clinical Trial Education: Of those treated by a gen heme/onc, 31% received no education on clinical trials. Only 15% reported good understanding of trial opportunities vs. 52% treated by a CLL expert (p<.001). 9% of pts treated by gen heme/onc were offered clinical trial participation vs. 47% treated by a CLL expert (p<0.001). Pts who declined or would have declined participation cited reasons including preference for "proven" tx (38%), distance from trial site (29%), fear (20%), frequent imaging (20%), overall lack of understanding about trials (15%), and lack of information (14%).
Summary: Most respondents want to be and are involved in tx decisions. While providers are influential in decision making, CLL pts also rely on outside sources. Gender, age, and tx status influence which factors drive decision making. Pts select tx based on response rate, survival, side effects, and ability to achieve deep response and want a plan in place for sequencing tx. The majority are willing to consider long-term tx without cure. Few want chemotherapy, CAR-T, or BMT unless they have no other options. A significant opportunity for improvement in education on clinical trial opportunities was identified. This description of pt experience, values, and preferences enriches the informed consent process and may lead to more tailored and patient-centered care.
Kennard:AbbVie, Gilead, Verastem: Consultancy. Furman:TG Therapeutics: Consultancy; Loxo Oncology: Consultancy; Sunesis: Consultancy; Verastem: Consultancy; Incyte: Consultancy, Other: DSMB; Genentech: Consultancy; Acerta: Consultancy, Research Funding; Gilead: Consultancy; Janssen: Consultancy; Pharmacyclics LLC, an AbbVie Company: Consultancy; AbbVie: Consultancy. Pagel:Pharmacyclics, an AbbVie Company: Consultancy; Gilead: Consultancy. Davids:Sunesis: Membership on an entity's Board of Directors or advisory committees; Roche: Consultancy; Astra-Zeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Research Funding; Genentech: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Astra-Zeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; MEI Pharma: Consultancy, Research Funding; Surface Oncology: Research Funding; Gilead: Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy; Merck: Consultancy; Merck: Consultancy; MEI Pharma: Consultancy, Research Funding; TG Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Astra-Zeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; MEI Pharma: Consultancy, Research Funding; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy; Sunesis: Membership on an entity's Board of Directors or advisory committees; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees; TG Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Genentech: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pharmacyclics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Gilead: Membership on an entity's Board of Directors or advisory committees; Verastem: Consultancy, Research Funding; Surface Oncology: Research Funding; Gilead: Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pharmacyclics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy; Roche: Consultancy; Pharmacyclics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees; BMS: Research Funding; Verastem: Consultancy, Research Funding; BMS: Research Funding; Genentech: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Surface Oncology: Research Funding; Verastem: Consultancy, Research Funding; TG Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Sunesis: Membership on an entity's Board of Directors or advisory committees; Merck: Consultancy; Roche: Consultancy. Nabhan:Cardinal Health: Employment, Equity Ownership. Kay:Acerta: Research Funding; Tolero Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees; Morpho-sys: Membership on an entity's Board of Directors or advisory committees; Pharmacyclics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Agios Pharm: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees; Gilead: Membership on an entity's Board of Directors or advisory committees; Infinity Pharm: Membership on an entity's Board of Directors or advisory committees; Cytomx Therapeutics: Membership on an entity's Board of Directors or advisory committees. Siddiqi:Juno Therapeutics: Other: Steering committee. Brander:Genentech: Consultancy, Honoraria, Other: Institutional research funding for non investigator initiated clinical trial, Research Funding; Pharmacyclics, an AbbVie Company: Consultancy, Honoraria, Research Funding; AbbVie: Consultancy, Honoraria, Other: Institutional research funding for non investigator initiated clinical trial, Research Funding; Teva: Consultancy, Honoraria; Novartis: Consultancy, Other: DSMB; TG Therapeutics: Consultancy, Honoraria, Other: Institutional research funding for non investigator initiated clinical trial, Research Funding; BeiGene: Other: Institutional research funding for non investigator initiated clinical trial, Research Funding; Acerta: Other: Institutional research funding for non investigator initiated clinical trial, Research Funding; DTRM: Other: Institutional research funding for non investigator initiated clinical trial, Research Funding. Wierda:AbbVie, Inc: Research Funding; Genentech: Research Funding. Hill:Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Seattle Genetics: Honoraria, Membership on an entity's Board of Directors or advisory committees; Genentech: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Seattle Genetics: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pharmacyclics: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Research Funding; Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pharmacyclics: Honoraria, Membership on an entity's Board of Directors or advisory committees. Cheson:AbbVie, Roche/Genentech, Pharmacyclics, Acerta, TG Therapeutics: Consultancy. Choi:Pharmacyclics: Consultancy, Research Funding, Speakers Bureau; Gilead: Speakers Bureau; Rigel: Consultancy; Genentech: Speakers Bureau; AbbVie, Inc: Consultancy, Speakers Bureau. Mato:AstraZeneca: Consultancy; Prime Oncology: Honoraria; Portola: Research Funding; Johnson & Johnson: Consultancy; Medscape: Honoraria; Celgene: Consultancy; TG Therapeutics: Consultancy, Research Funding; Acerta: Research Funding; Pharmacyclics, an AbbVie Company: Consultancy, Research Funding; AbbVie: Consultancy, Research Funding; Regeneron: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal